Remote Rapid COVID-19 Serology (Antibody) Testing Availability
5/8/2020
Document Number 1
We would like to announce that we are currently set-up to provide highly accessible, convenient testing for COVID-19 serum antibody (as well as nasal viral PCR swab test; see elsewhere). The presence of this antibody suggests resolved infection and probable immunity from re-infection (how long is unknown, though likely at least a couple years, if not lifelong). How protective this antibody is against re-infection and the duration of that protection (which will likely vary from person to person) will be defined in the next few years, but currently is unknown.
No appointment with the doctor is needed. We will immediately set-up a medical requisition for you at a lab for the bloodraw.
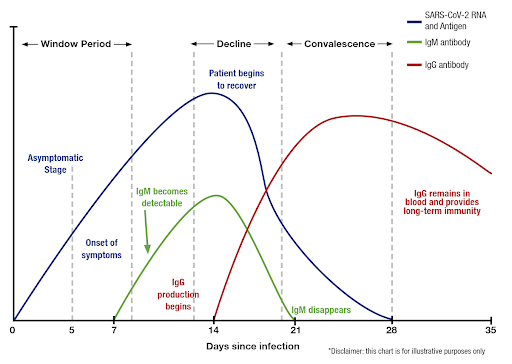
When results are back, we will notify you and provide documentation of the result for presentation to any interested parties – such as nursing home staff, hospital staff, employers or any other entities who need to know that you do not have COVID-19 and are immune to it. It is best to do this test at least 2 weeks after the onset of COVID-19 illness, to minimize the chance that you might still be shredding dangerous virus as your infection resolves. The antibody test cannot determine whether you are colonized with or shedding infectious virus, but it is unlikely once you are IgG (late) antibody positive.
If you are getting tested post recent COVID-19 or suspected COVID-19 illness, you should continue to practice infection control measures (distancing, masks, washing) for at least 1 week after the blood was drawn or 3 weeks after last illness symptoms to be sure you had not just gotten over the infection as the blood was drawn, since infected individuals may continue to shed COVID-19 virus at least 3 weeks post-infection onset. Up to 80% of people infected with COVID-19 have no or minimally noticeable symptoms.
Please do NOT call our office after hours- Our answering service is for emergencies. If you have questions, please call between 9 am – 3pm
**OR** send us your intake form following the procedure below- Our staff will call you promptly!
PROCEDURE
- To start the testing information intake process, go to our website’s COVID-19 WALK-IN TESTING INTAKE FORM ORDER SHEET (click on red sentence below).
- Payment will be required prior to or at the time of our ordering your COVID-19 blood test and the service charge will not be billed to medical insurance. The laboratory’s test charge itself may be covered, but you will need to provide your medical insurer with the cost of the test for possible reimbursement.
- We will provide written documentation of your result and an explanation of any possible implications of the result for you as soon as it is available. We suggest you take a photo of that document to store in your mobile phone, just like a driver’s license
Note: The RELIABILITY of current COVID-19 antibody tests has NOT BEEN ESTABLISHED. If you decide to proceed with testing, you accept this limitation on the significance of the result. For a more detailed explanation on the state-of-the art on COVID-19 antibody testing and what it can and cannot achieve, see “COVID-19 Antibody (Serum) Testing”.
Edward R. Rensimer, MD
Director, International Medicine Center
CORONAVIRUS-19 WALK-IN TESTING INTAKE FORM ORDER SHEET (CLICK HERE)
Remote COVID (SARS-CoV-2) ANTIBODY Testing
International Medicine Center
9230 Katy Freeway, Suite 400
Houston, TX 77055
713-973-6078
imc@traveldoc.com
5/8/2020
Document Number 2
Our facility is offering expedited, remote (no physician visit needed) testing for SARS-CoV-2 antibody. We prefer to do this without appointment so that we can manage workflow in our facility as well as to minimize exposure of all involved to each other. The aim is maximal testing of the community with minimal waste of time and exposure to others. See our detailed comments on the value and limitations of the test itself in Document Number 3.
PROCEDURE,
1. To receive a testing order by our physician, you must complete and email us our short testing request form,
CORONAVIRUS-19 WALK-IN TESTING INTAKE FORM ORDER SHEET (CLICK HERE)
Send to: imc@traveldoc.com
2. We will call you to confirm that a test order has been sent to the lab or to schedule you a brief office visit for the nasal swab test.
3. When results are back, we will call you,
Result Turnaround Time:
Antibody: 1-3 days
Nasal Swab PCR: 2-7 days
4. If physician consultation on the results is desired, notify us so we can set up a formal appointment.
5. Charges: Administration/Case Evaluation Fee:
IgG Antibody Test: $50
PCR Test: $75
IgG Antibody Test Charge: $50 LabCorp; $50 Quest Lab
PCR Test Charge: $100 LabCorp; $100 Quest
We can provide the insurance to the lab for them to bill, but we cannot guarantee this test will be covered by your insurer.
We will notify you of the result, and provide a certification of result document which will provide interpretation and possible implications of the result.
Edward R. Rensimer, MD
Director
International Medicine Center
CORONAVIRUS-19 WALK-IN TESTING INTAKE FORM ORDER SHEET (CLICK HERE)
COVID-19 Antibody (Serum) Testing
5/8/2020
Document Number 3
There is overwhelming demand for the antibody test for Coronavirus-19 (SARS-CoV-2). People want to know if they can go back to work, their kids see their friends, go to restaurants and sports events… get their lives back. There is a building sense that we have an economic emergency as, if not more, important than the pandemic. After all, the pandemic largely targets mostly limited groups of people for severe illness or death – the elderly and the medically compromised. But, the economic disaster hits everyone and potentially could do so for years. We need this test.
But, the urgent need does not create a reliable test, at least not yet. What it does is create an entrepreneurial opportunity to be exploited by companies looking to get rich on the desperation of everyone hoping to end the public health shut-down and to start companies up again. We all want this.
But, does desperation ever result in anything but serious, perhaps grave mistakes? What does a test need in order to be reliable? Think about it. What would it mean to you if a pregnancy test were unreliable, with a high rate of false positives? What about a cancer screening test with a high rate of false positives or false negatives? In other words, you could not be near sure that you did or did not have cancer, despite the result your physician gave to you.
That is the reason the federal Food and Drug Administration (FDA) evaluates new medical tests. It determines whether a test reliably yields results that are ultimately true to the purpose of the test.
In the context of a national emergency of contagious disease, the FDA has decided to allow tests for COVID-19 antibody to be offered to the public as EUA, Emergency Use Authorization. If the tests APPEAR to be legitimate, they get an EUA status. But, such tests have not been studied for sensitivity and specificity by the FDA– how well they detect COVID-19 antibodies and whether those antibodies are a result of the COVID-19 virus, rather than other coronaviruses, which cause 20-30% of head colds. This is called “cross-reactivity”, leading to false positives for SARS-CoV-2 virus antibody reports. Currently available tests have not been vetted by the FDA for tendency to cross-reactivity for coronaviruses other than that which causes COVID-19 disease.
So, where are we?
COVID-19 antibody testing is being offered here and there. Is it reliable?
Well, many of the antibody test companies have products made in China. At this point, there are over 120 companies in the game. Some have claimed 80% sensitivity (8 out of 10 chances if you have the antibody their test will be positive). But, in use, some of their tests have actually only been 20% positive. Is that a reliable test? And so, at least 20% of the people will at least know they have the antibody, right? No. WHEN THERE IS LOW PREVALENCE OF A DISEASE ACROSS A LARGE NUMBER OF PEOPLE, ANY POSITIVE IS LIKELY A FALSE POSITIVE.What does that mean? If I test 1000 2 year-old baby boys for pregnancy, and 3 tests are positive, are 3 baby boys pregnant? Or, is the test false positive because of something else in those boys’ bloodstream? If I test 1,000 20 yr-old, sexually active women for pregnancy and 3 are positive, what are the chances those 3 girls are pregnant? Virtually 100%. The prevalence of pregnancy in baby boys is zero. The prevalence of pregnancy in 20 year-old women is substantial. The prevalence of the problem the test looks for in a given population determines how likely a positive is true or false.
So far, screening in large populations here and in other countries is suggesting 3-5% of the general population had been COVID-19 exposed by positive antibody tests. That means any positive antibody test is more than likely false positive. That problem will hopefully be solved by antibody tests that have passed muster for sensitivity and specificity, and so achieve FDA-APPROVAL (not “authorized”)
With the currently available antibody tests, this is IDEALLY (if the specific testing product ultimately meets medical standards) what the antibody tests CAN DO,
1. Tell who has been infected and developed an immune response to COVID-19 virus.
2. Tell the POSSIBILITY of some (uncertain how much or how long) immunity to COVID-19.
3. False Negative Result: the individual has been infected, but has negative antibody because,
a. Weak immune system due to underlying medical conditions or medications (cannot produce antibodies)
b. The specific testing product (brand) is not of good quality.
c. The individual is actually infected and it is too early in the illness to form antibodies.
4. True Negative: the only solid valuable result; the person is still at risk for infection
5. Tell who qualifies to donate plasma for possible COVID-19 investigational treatment with antibodies
Ideally, what the current antibody tests CANNOT DO,
1. Tell if a person is immune to re-infection. It is unknown whether the antibodies detected by currently available tests are “signal” (just “flags” that the virus has passed through a body) or “protective”. People with Herpes simplex have positive serum antibodies, yet they have recurrent herpes attacks. Immunity against any infectious agent can be complex; more than just due to antibodies.
2. Tell how long the antibodies will last
3. Diagnose or exclude active COVID-19 infection
What do you need to know?
1. If an antibody test is performed at home, an urgent care center, or a doctor’s office (usually “rapid” tests), it is likely unreliable. Commercial labs, like LabCorp and Quest, will offer test products more likely to eventually receive FDA-approval status, and so with results that may be more reliable, though that is still not established.
2. No one knows if COVID-19 antibodies are protective against re-infection (though, likely they are, at least partly) or how long they last.
3. Antibody testing done by large commercial labs, such as LabCorp and Quest, are probably the best tests available at this time, though still may be far from the high standards for any medical testing (ideally sensitivity and specificity over 95%, with a very low rate of false positives).
4. Regardless of how good any antibody test product is, the science is not yet developed on whether the antibody detected by the test can imply immunity to SARS-CoV-2 virus. The result cannot be used to decide on the need (or lack of it) for continued quarantine, social distancing, masks, etc.
CONCLUSION: Beware of antibody testing and relying on the results at this time. Currently, the World Health Organization, given the COVID-19 antibody tests available, recommends against it.
At this time, we can offer this testing, provided the patient/client is fully informed on all the issues described in this document
AS SOON AS SUCH TESTING CAN BE RELIED ON, WE WILL PROMOTE IT.
Edward R. Rensimer, MD
Director, International Medicine Center
Houston, TX
CORONAVIRUS-19 WALK-IN TESTING INTAKE FORM ORDER SHEET (CLICK HERE)
ON-SITE, REMOTE LOCATION COVID-19 GROUP TESTING SERVICE
Rensimer & Associates can, on a case-by-case basis, depending upon location, number of tests, and time of day/urgency, offer remote location testing of groups.
1. Physician Time: $450/hour*
Staff Time: $75/hour/staff member (usually two support staff needed)*
Administrative fee: $50/test
Test Fee (nasal swab):
LabCorp $100
Quest $100
*Travel time will be billed
These fees will be paid up front with reimbursement for tests not done if the final total of tests is less than the number of tests previously projected. Physician and staff time fees will not be refunded. Minimal charge for our time will be $1,000.
2. This service includes patient results callback, results reporting to Public Health Dept., and a certificate of test results.
3. A group listing of examinee names, phone numbers, and dates of birth will be required 24-48Hrs prior to an appointment to minimize time needed to provide the service, and so your lower cost.
4. To fairly estimate the cost, we project about 90 min/50 tested individuals at your location if all information has been provided upfront and the individuals are immediately available.
5. Documents Provided,
COVID-19 POSITIVE RESULTS ACTION FORM: provides directions to the examinee on steps to take if they test positive.
COVID-19 POINT OF VIEW/BUSINESS: This is a document that provides fact-based information to employees relevant to their return to the workplace during the COVID-19 pandemic. The document can be modified to the needs of the organization.
Edward R. Rensimer, MD
Infectious Diseases
Director, International Medicine Center
Copyright, 2020, E. R. Rensimer, MD, All Rights Reserved
